EORTC QLQ: Measuring Quality of Life in Cancer
The EORTC QLQ helps researchers and doctors assess the quality of life in cancer patients. This self-report tool tracks physical and emotional health to support better care.


European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) assesses health-related quality of life in cancer patients. Using a core survey plus specific modules, it helps clinicians track physical, emotional, and social functioning to support better care decisions.
Category
Disease
Source
What is European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)

The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) is a trusted tool for checking patient wellbeing. It relies on a central set of thirty questions known as the QLQ-C30 mixed with specific modules for different tumour types to capture the whole clinical picture accurately. Patients rate their health across physical, emotional, and social areas while tracking common symptoms like fatigue or pain. Most items use a simple range from not at all to very much so it is straightforward for people to answer honestly without any confusion. Doctors use this data to make better treatment choices in clinical trials and daily practice. The modular setup helps clinicians focus on specific issues without overwhelming the patient, making it a smart pick for tracking progress and adjusting care plans effectively over the long haul.

The EORTC QLQ relies on a standard linear transformation method to score its various scales. First, you figure out the average of the items in each scale to get a raw score. Then, use the official manual to convert these numbers onto a 0 to 100 range. It is worth noting that a high score on functional scales means you are doing well, while a high score on symptom scales points to more health troubles. This clear difference helps doctors track how a patient is coping with cancer treatment over time.

Advantages
Strengthens evidence through systematic measurement approaches.
Applicable across multiple diseases and conditions.
Meets international clinical assessment requirements.
Measures healthcare intervention results systematically.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Collect EORTC QLQ data with WeGuide, the all-in-one patient engagement platform
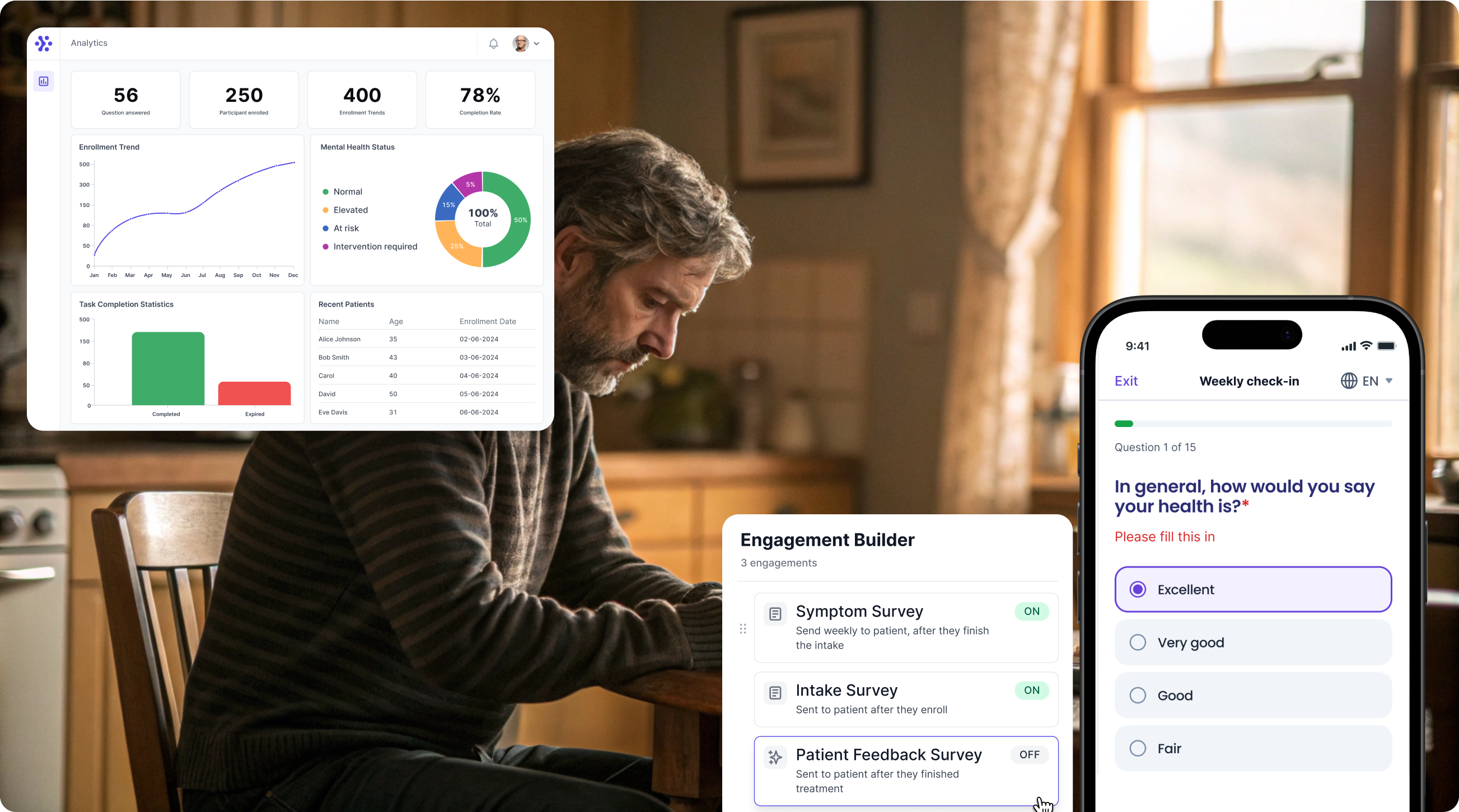

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




