EORTC QLQ-C30: Measuring Cancer Quality of Life
The EORTC QLQ-C30 helps doctors assess the quality of life in people living with cancer. This survey tracks physical and emotional health to improve patient care during treatment.


EORTC QLQ-C30
Developed by the European Organisation for Research and Treatment of Cancer, the EORTC QLQ-C30 assesses quality of life. This 30-item questionnaire tracks physical, emotional, and social functions alongside symptoms. It's a key tool for monitoring cancer patients in clinical trials and routine practice.
Category
Disease
Source
What is EORTC QLQ-C30

The EORTC QLQ-C30 is a trusted tool for checking the quality of life in cancer patients. It helps doctors understand a patient's health journey better. This thirty-item questionnaire covers a broad range of cancer-specific issues. It mixes multi-item scales with single items to capture the full picture of a patient's status. It looks at five functional areas like physical and emotional health plus symptom scales for fatigue and pain. Most questions use a simple one-to-four scale, while the overall health rating uses a one-to-seven range so patients can easily share exactly how they feel. Clinicians use this tool in trials and daily practice to track changes over time. It is reliable and valid across many cultures. By spotting issues early, healthcare teams can adjust treatments to support patient wellbeing and ensure that care plans truly meet the specific needs of the individual.

The EORTC QLQ-C30 is scored using a standardised method that converts raw answers into a 0 to 100 scale. You start by averaging the items within each scale to find the Raw Score. Then, use a linear transformation formula to shift these numbers to a 0 to 100 range. It is crucial to watch the direction here. For functional scales and global health status, a higher score means better function. However, for symptom scales like pain or fatigue, a higher score points to more severe problems. This clear distinction helps doctors spot changes in a patients quality of life during cancer care.

Advantages
Strengthens evidence through systematic measurement approaches.
Meets international clinical assessment requirements.
Serves diverse clinical needs effectively.
Measures healthcare intervention results systematically.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Collect EORTC QLQ-C30 data with WeGuide, the all in one patient engagement platform
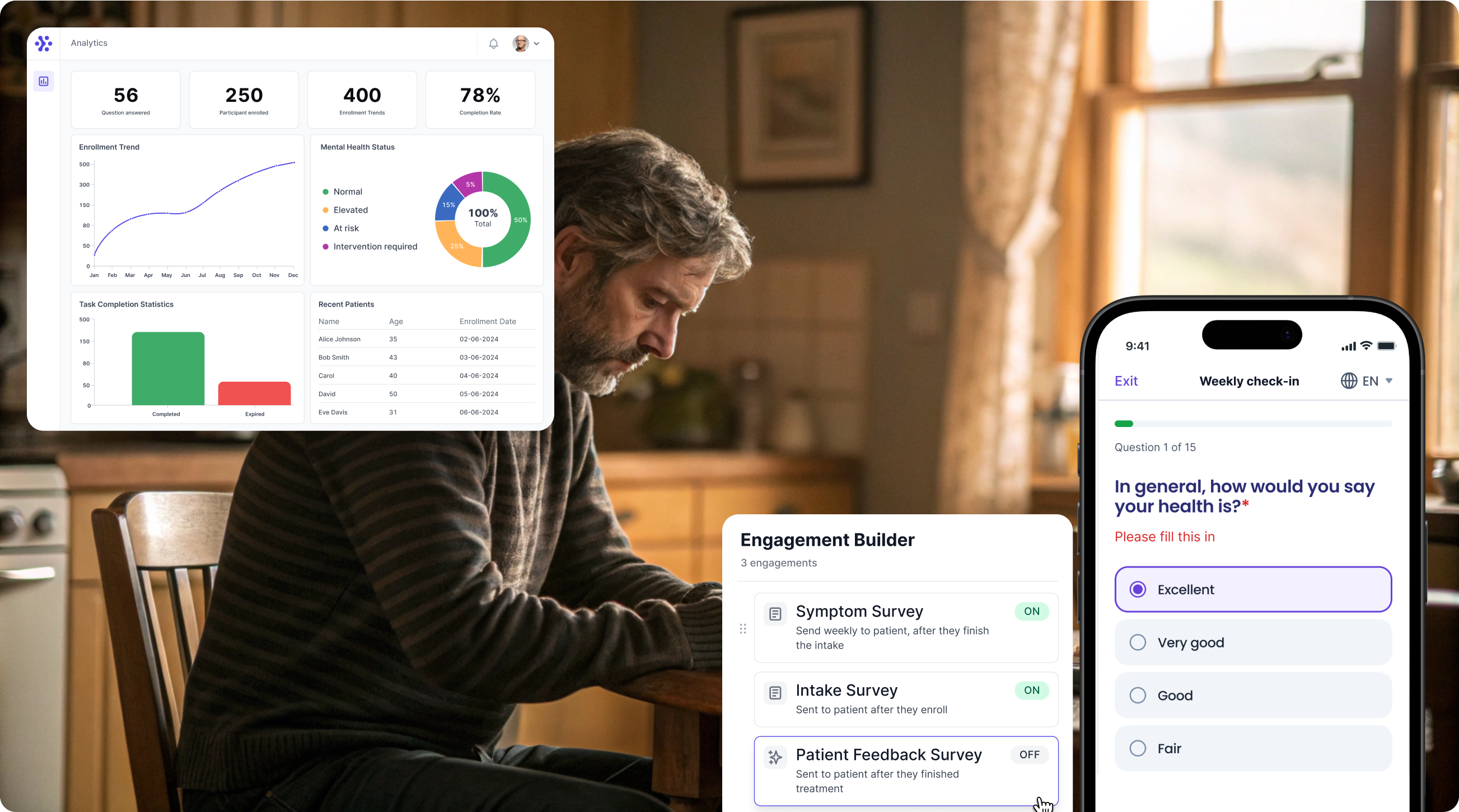

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




