The Eating Disorder Examination Questionnaire (EDE-Q)
The EDE-Q helps clinicians assess eating disorder behaviours and attitudes through a simple form. It allows doctors to track symptom severity and monitor progress throughout a treatment plan.


Eating Disorder Examination Questionnaire (EDE-Q)
The Eating Disorder Examination Questionnaire (EDE-Q) is a key self-report tool for assessing eating behaviours. Focusing on the past 28 days, it measures restraint plus concerns regarding eating, shape, and weight. It’s essential for screening and monitoring treatment progress in clinical care.
Category
Disease
Source
What is Eating Disorder Examination Questionnaire (EDE-Q)

The Eating Disorder Examination Questionnaire (EDE-Q) is a trusted self-report tool for assessing eating disorder behaviours and attitudes. Derived from the standard interview format, this instrument focuses on the past 28 days to help clinicians understand the severity of specific psychopathology in depth. The scale generates a global score alongside four subscales: Restraint, Eating Concern, Shape Concern, and Weight Concern. It also tracks the frequency of key behaviours like binge eating or purging to provide a clearer picture of complex symptoms. It’s quick to administer and score, making it great for busy clinics. Researchers often use it to track treatment progress over time. It offers a practical way to screen for issues like anorexia or bulimia without needing a long interview, ultimately supporting better care planning for your clients.

The EDE-Q can be scored using two main approaches: calculating subscale scores and deriving a global score. 1. Subscale Scores: You average items rated 0 to 6 across four areas: Restraint, Eating Concern, Shape Concern, and Weight Concern. This helps pinpoint specific struggle areas. 2. Global Score: You average the four subscale totals to get a single number representing overall severity. Clinicians also tally the frequency of key behaviours like bingeing separately to check for immediate risks. It is a practical way to see how a patient is tracking throughout their recovery journey.

Advantages
Streamlines research data gathering process.
Measures healthcare intervention results systematically.
Enables precise identification of patient health status.
Meets international clinical assessment requirements.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Collect EDE-Q data with WeGuide, the all in one patient engagement platform
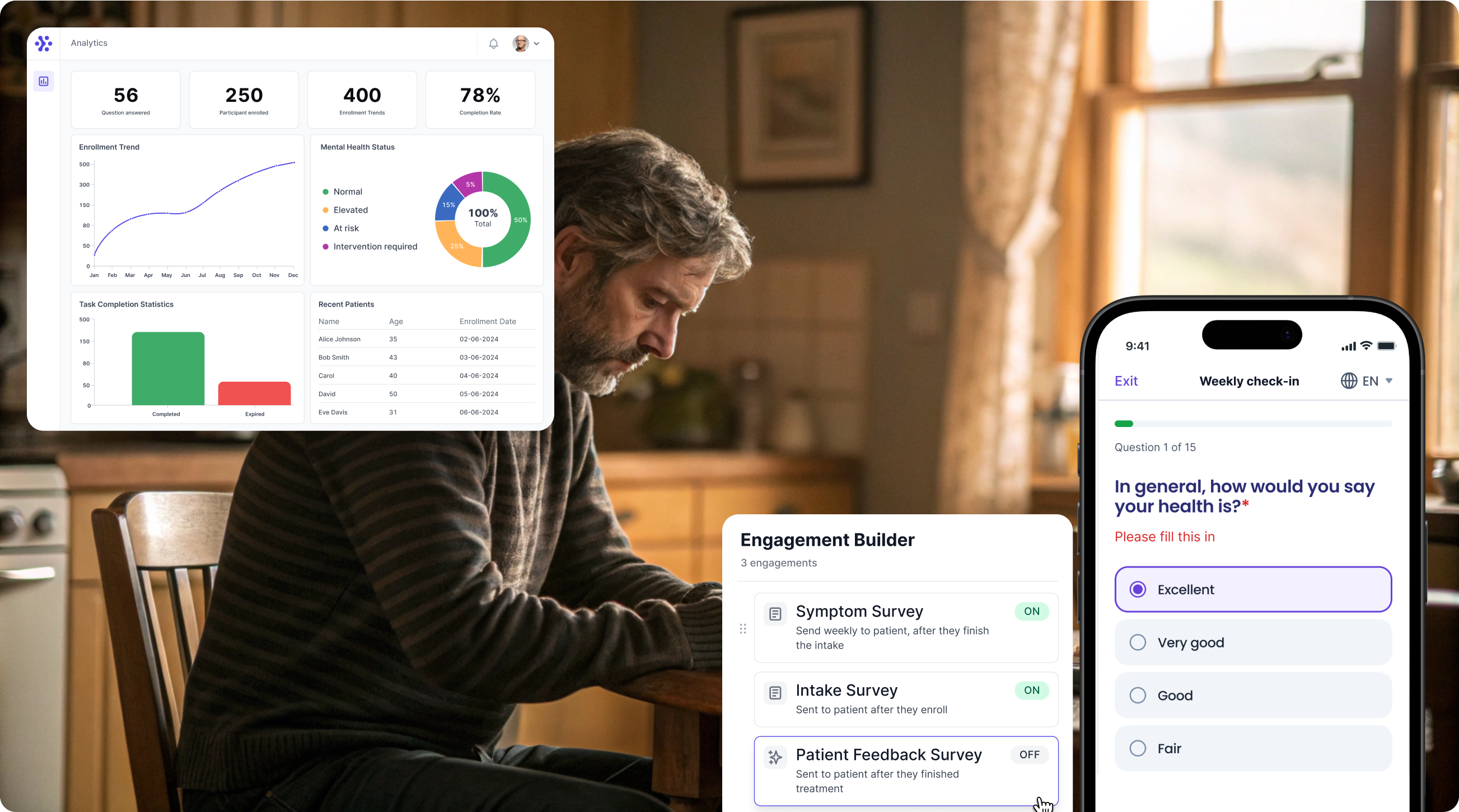

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




