Disability Rating Index (DRI): Measuring Physical Function
The Disability Rating Index is a self-report tool measuring physical disability from musculoskeletal pain. It helps clinicians track how conditions affect daily tasks like dressing and lifting.


Disability Rating Index (DRI)
The Disability Rating Index (DRI) is a self-administered questionnaire designed to assess physical disability levels. Patients use it to rate their ability to handle daily physical activities, such as lifting or climbing stairs. It’s a practical tool for monitoring recovery in musculoskeletal conditions.
Category
Disease
Source
What is Disability Rating Index (DRI)

The Disability Rating Index (DRI) is a handy tool for measuring physical disability. It helps clinicians track functional health in pain patients. This questionnaire covers twelve specific items related to daily physical activities. It focuses on movements that are often hard for people with common musculoskeletal issues. Patients rate their ability to perform tasks like dressing, climbing stairs and running on a visual analogue scale. They mark a line between zero meaning no difficulty and one hundred representing total inability to perform the task at all. Since it is self-administered, the DRI is easy to use in busy clinics or rehab settings. It works well for assessing long-term neck and back pain. Doctors value it because it spots small changes over time, making it a reliable choice for planning treatment and checking recovery progress effectively.

The Disability Rating Index can be scored using a single measurement based approach. Patients mark a 100mm line for 12 daily activities like dressing or lifting. One end means no trouble at all while the other means you cannot do it. To score it, you measure the distance from the zero point in millimetres. Add these up and divide by 12 to get the mean. The final result sits between 0 and 100. Higher numbers suggest more physical trouble, giving clinicians a clear picture of impairment levels in daily life.

Advantages
Measures healthcare intervention results systematically.
Simple to understand and complete for patients.
Provides consistent measurements for clinical research.
Serves diverse clinical needs effectively.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Collect Disability Rating Index (DRI) data with WeGuide, the all in one patient engagement platform
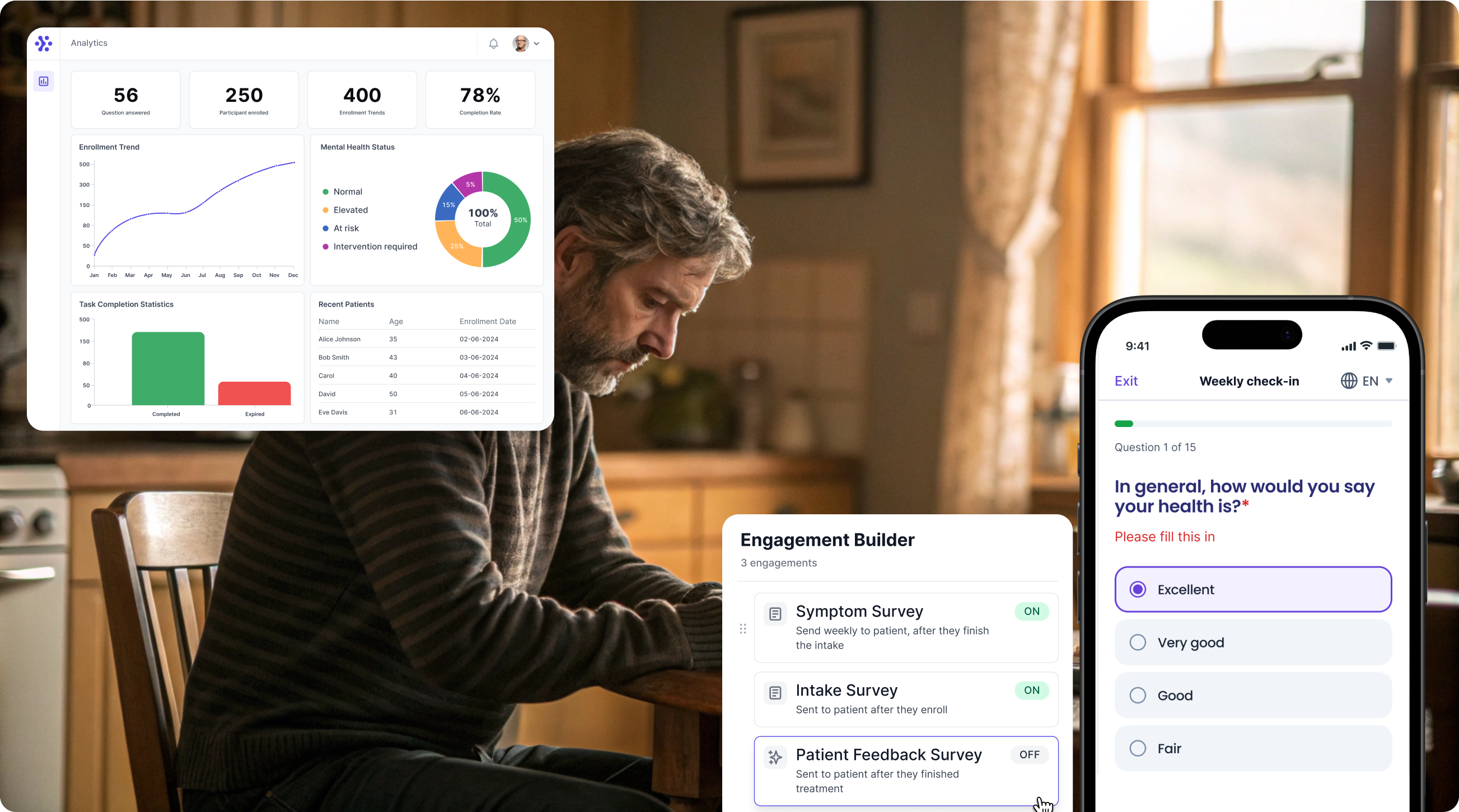

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




