The Center for Epidemiologic Studies Depression Scale
This tool assesses current depression symptoms across the general population. Researchers use it to monitor mental health trends and identify people who may require professional support.


Center for Epidemiologic Studies Depression scale
The Center for Epidemiologic Studies Depression scale (CES-D) is a short self-report tool used to measure depressive symptoms in the general population. With 20 items assessing feelings over the past week, it's a reliable way to screen for depression in research and clinical settings.
Category
Disease
Source
What is Center for Epidemiologic Studies Depression scale

The Center for Epidemiologic Studies Depression scale, or CES-D, is a trusted tool for checking depressive symptoms in the general population. This self-report survey features 20 simple items that ask about how you have felt over the past week. It looks at current feelings instead of a full clinical diagnosis. Participants rate statements on a four-point scale ranging from rarely or none of the time to most or all of the time. The questions cover key areas like restless sleep, poor appetite, feelings of loneliness, and general sadness to build a clear picture of your mental state. It is really handy for researchers and clinicians because it is quick to finish in about five to ten minutes. While it does not diagnose depression on its own, it is a top choice for screening large groups or tracking mood changes over time. This makes it a practical option for many community health studies.

The Center for Epidemiologic Studies Depression scale can be scored using one main approach: total summation. First, assign values from 0 to 3 for each of the 20 items based on frequency of feelings. You'll need to flip the scores for the four positive questions so higher numbers reflect more depressive symptoms. Once that is sorted, add everything up to get a total between 0 and 60. A result of 16 or more typically flags a risk of clinical depression, giving clinicians a clear sign to investigate further.

Advantages
Strengthens evidence through systematic measurement approaches.
Streamlines research data gathering process.
Serves diverse clinical needs effectively.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Streamline Center for Epidemiologic Studies Depression scale assessments with WeGuide
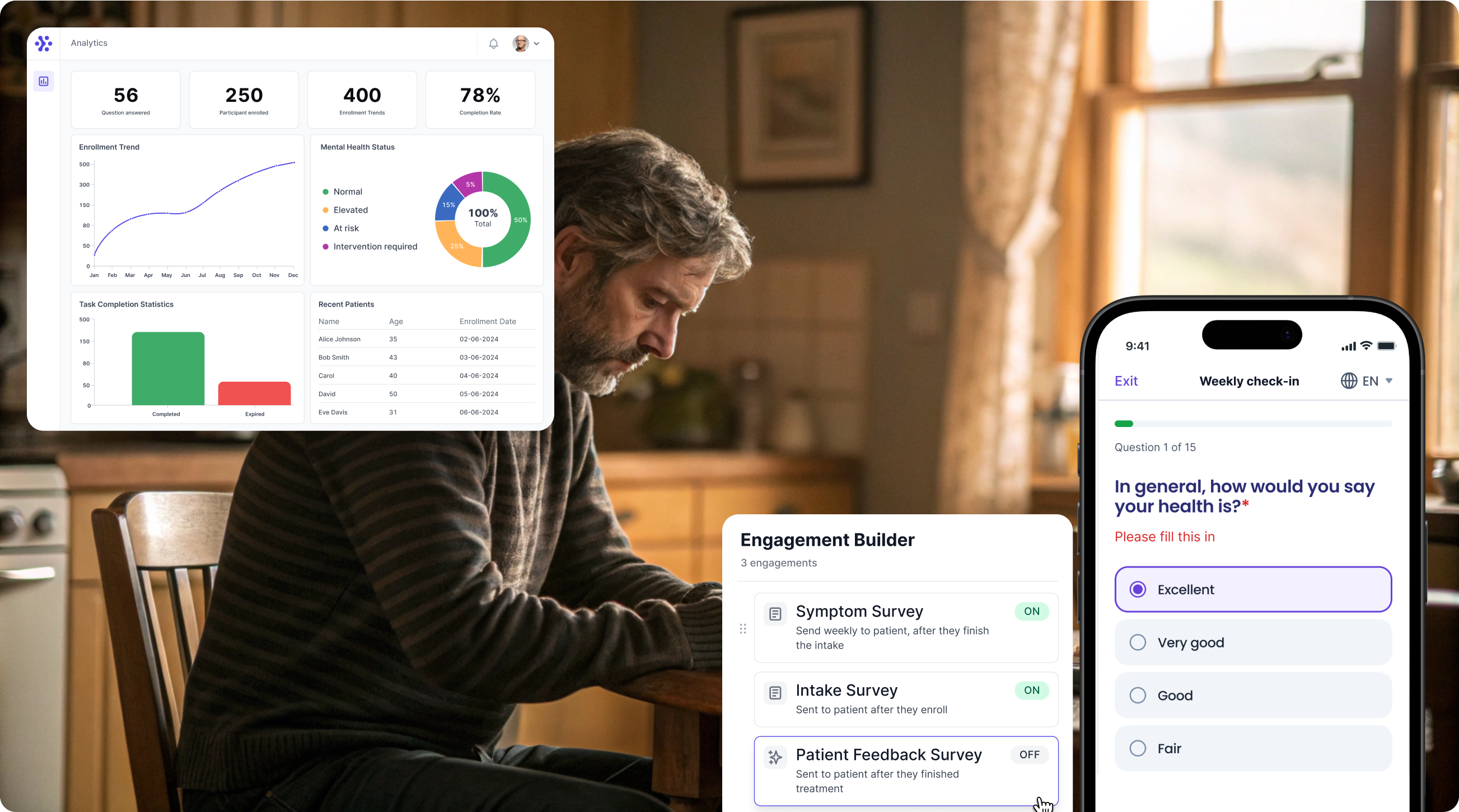

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




