Beck Depression Inventory: Measuring Depression Severity
The Beck Depression Inventory allows clinicians to measure the severity of depression through a simple self-report questionnaire. It helps track symptom changes and guides treatment decisions.


Beck Depression Inventory
The Beck Depression Inventory (BDI) is a 21-item self-report scale measuring characteristic attitudes and symptoms of depression. It helps healthcare professionals assess the severity of depressive symptoms, serving as a standard tool for mental health screening and monitoring progress.
Category
Disease
Source
What is Beck Depression Inventory

The Beck Depression Inventory (BDI) is a widely used tool for measuring depression severity. It helps clinicians track mood changes effectively. This questionnaire has 21 items and looks at how someone is feeling right now. It covers emotional, physical, and cognitive symptoms that often point to major depression. Patients rate items on a scale from zero to three based on how they felt over the last two weeks. Questions explore sadness, hopelessness, guilt, and fatigue. It also checks for changes in sleep or appetite, giving a clear picture of symptom intensity. Taking just five to ten minutes to finish, it is easy to use in busy clinics or research settings. Doctors use the total score to decide on treatment plans or see if therapy is working. It's a reliable way to spot warning signs early and supports better mental health outcomes for every patient.

The Beck Depression Inventory can be scored using a single cumulative approach known as the Total Sum Score. 1. Calculation: This tool lists 21 symptom categories like mood or sleep. Patients pick a statement rating from 0 to 3 for each one. You just sum these up to find a total ranging from 0 to 63. 2. Clinical Use: Higher tallies suggest deeper depression levels. Health pros use standard cutoffs, where 14 to 19 might show mild issues and scores over 29 indicate severe depression, helping them sort out the best care plan.

Advantages
Enables precise identification of patient health status.
Measures healthcare intervention results systematically.
Streamlines research data gathering process.
Strengthens evidence through systematic measurement approaches.
Related Instruments
Assesses physical function of the foot and ankle.
Assesses the overall impact and severity of fibromyalgia.
Assesses key dimensions of female sexual function.
Streamline Beck Depression Inventory data collection with WeGuide
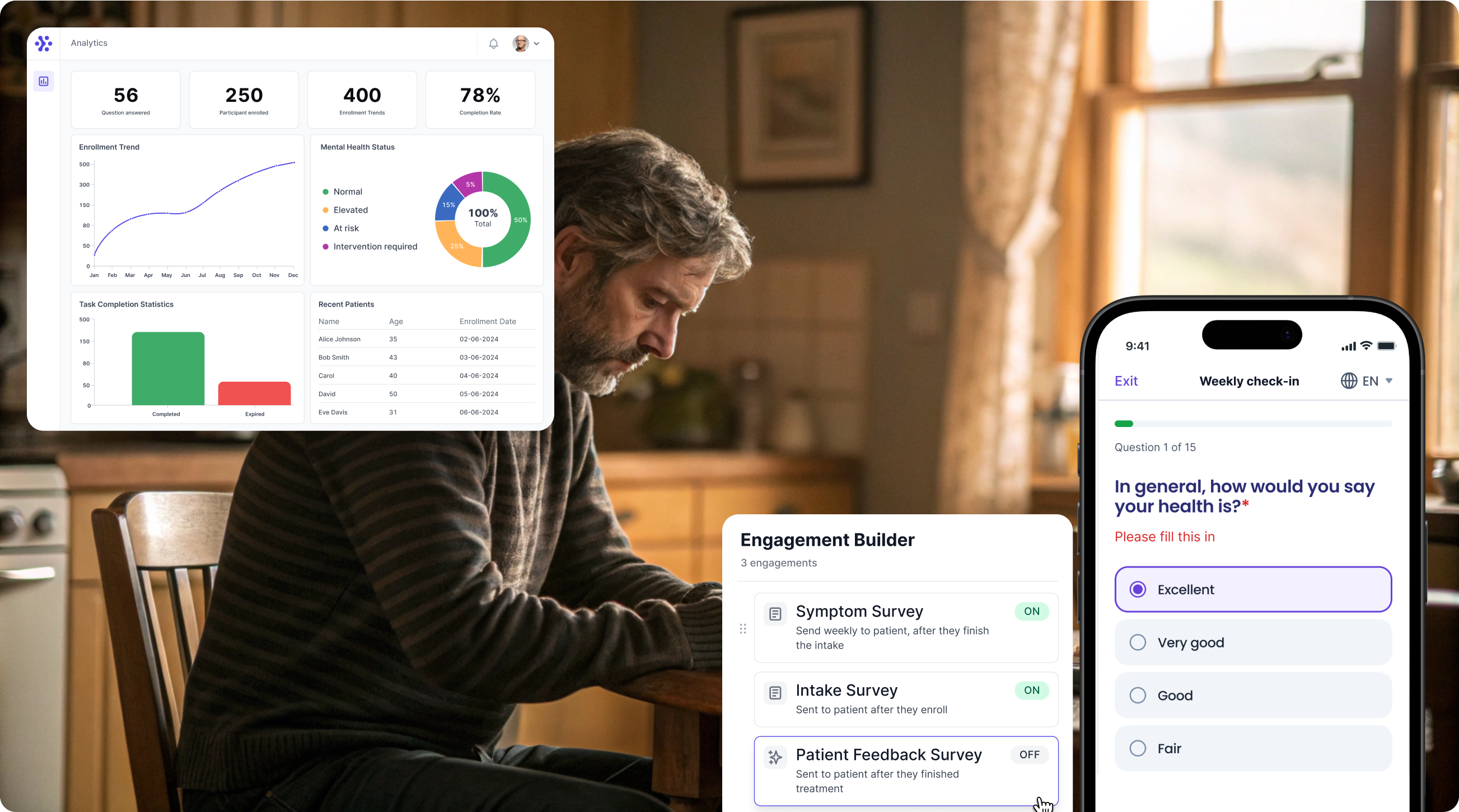

Support Your patient in no time - all under your own brand
Our platform combines the knowledge of 100+ digital health solutions built, letting you easily build your patient engagement app for research or clinical use. Collect PROMs, support patients on waiting lists, or gather vital signs data. This is how:
Learn MoreProgram Builder
.png)
Whitelabelling
.svg)
.svg)
Turn on components, dependent on your usecase
.svg)
Support The Full Journey
.png)
.png)
%20(1).png)
.png)
It’s your turn
Every WeGuide project, saves researchers and clinicians - 9 months and $450,000 - creating impactful digital health solutions.




